L. David Roper
http://www.roperld.com/personal/RoperLDavid.htm
2 July, 2016
With the certainty of peak crude oil, followed by peak natural gas, personal transportation faster than walking and bicycling must be by electric vehicles. The electrical storage for those cars will be either batteries or ultracapacitors or both.
Of course, batteries are made of minerals each of which will also have a peaked extraction curve similar to crude oil and natural gas. However, most of those minerals can be recycled, which is not the case for burned crude oil and natural gas. This study is to estimate how many cars with sizeable electrical-energy storage can be manufactured per year into the future.
It will be shown that, even with multiple recycling, eventually the minerals used to make electrical-energy-storage devices will be spread diffusely out over the Earth and will diminish with time such that no such devices can be made.
The recyling for the minerals is estimated from a mathematical theory of minerals recycling that I have developed.
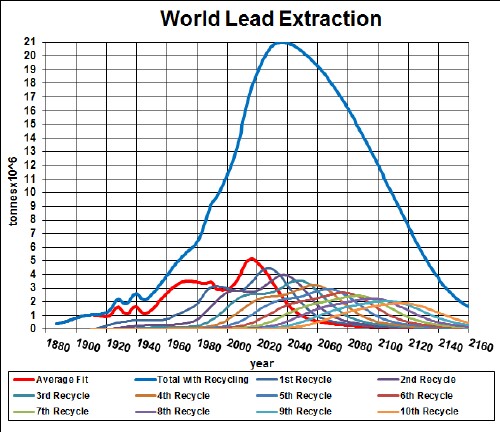
Lead-acid batteries (LAB) have been used for many years for energy storage for vehicles. New technologies have recently been developed for LABV; e.g., the Oasis Battery by Firefly Energy.
Here is a graph showing the result of my depletion analysis, including recycling, for lead:

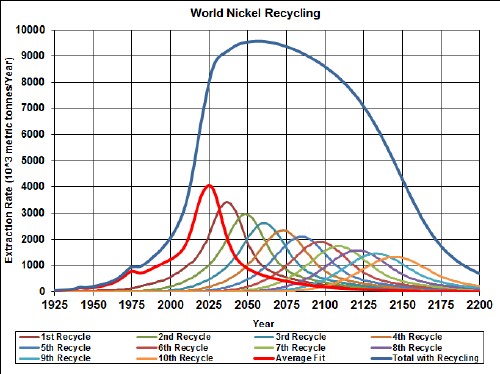
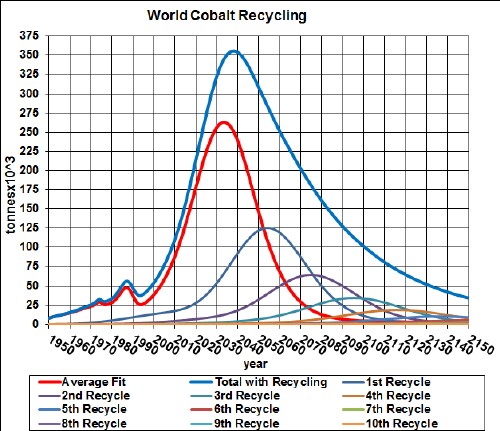
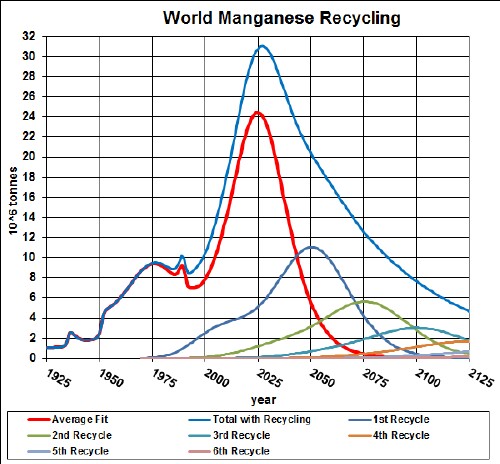
The first generation of hybrid (gasoline-electric) vehicles use nickel-metal-hydride batteries (NiMH). The minerals used are nickel, cobalt, manganese and rare earths.
Here are graphs showing the results of my depletion analyses, including recycling, for nickel, cobalt, manganese and rare earths:
 |
 |
 |
 |
The first mass-produced electric vehicles use lithium-ion batteries (LiIon). The minerals used are lithium, cobalt, manganese and phosphate.
Here are graphs showing the results of my depletion analyses, including recycling, for lithium and phosphate rock:
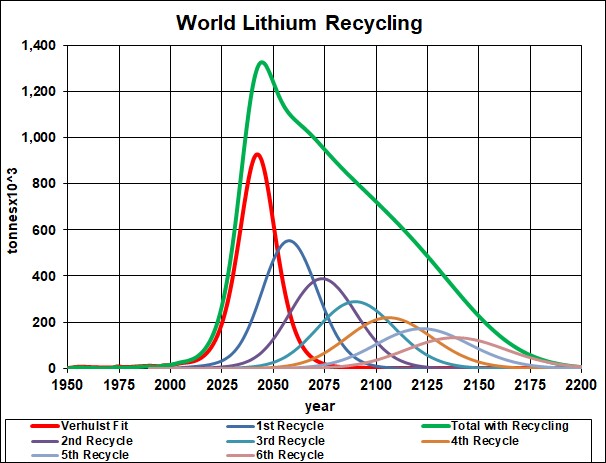 |
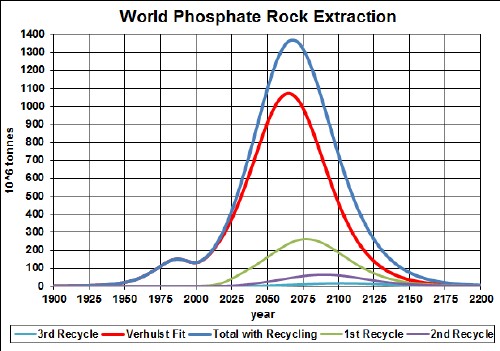 |
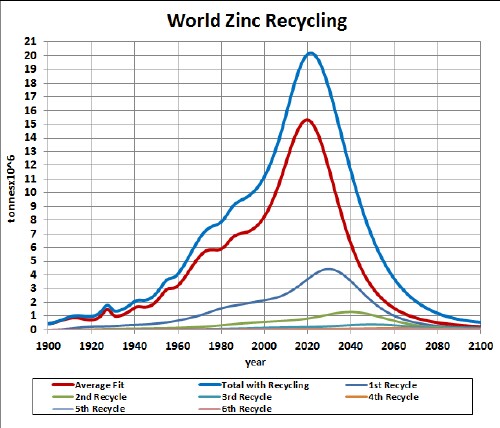
Zinc-air batteries (ZAir) are used in hearing aids and other small devices. Research is ongoing for using them in electric vehicles.
Here is a graph showing the result of my depletion analysis, including recycling, for zinc:
Although not batteries, ultracapacitors (UC) store electric energy. Their advantages over batteries are:
Their disadvantage compared to batteries is the amount of charge that can be stored. A new type of ultracapacitor (EESU=Electrical Energy Storage Unit) may overcome this disadvantage. So, I include UC in this study of batteries materials.
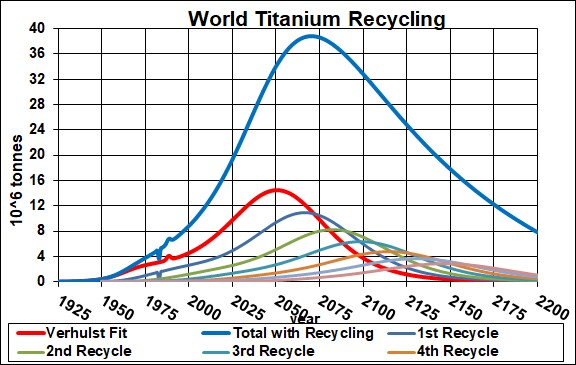
Minerals used to make the EESU are barium and titanium. Here are graphs showing the result of my depletion analysis, including recycling, for barite and titanium:
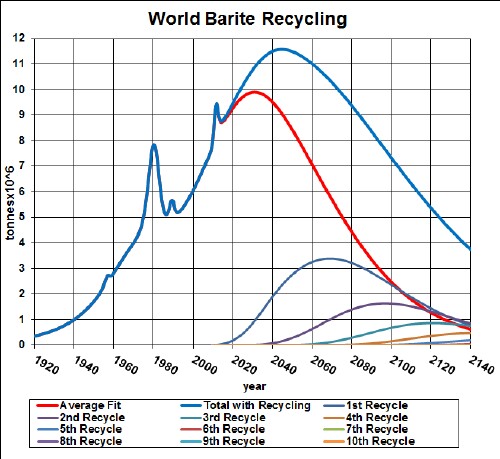 |
 |
The following table list the approximate depletion peaks, including recycling, for the major minerals used to make batteries (including ultracapacitors):
Mineral |
Peak Year |
lead |
2045 |
nickel |
2075 |
cobalt |
2065 |
manganese |
2050 |
rare-earths |
2090 |
lithium |
2075 |
phosphate |
2030 |
zinc |
2015 |
barite |
2000 |
titanium |
2045 |
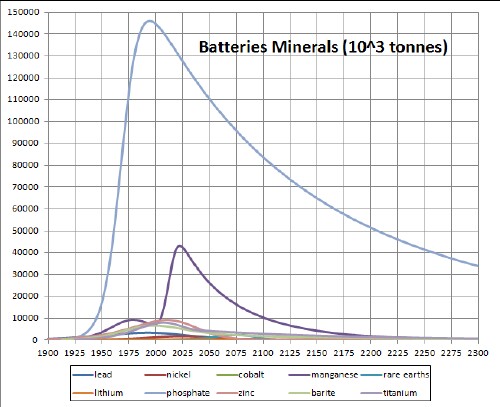
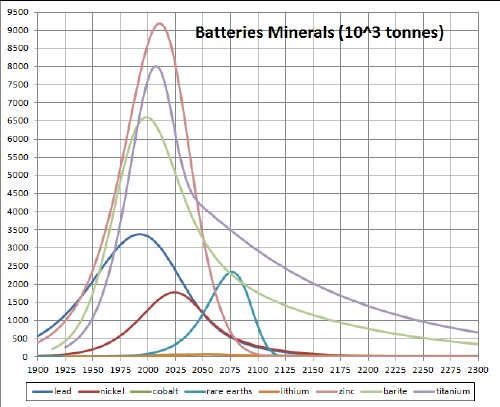
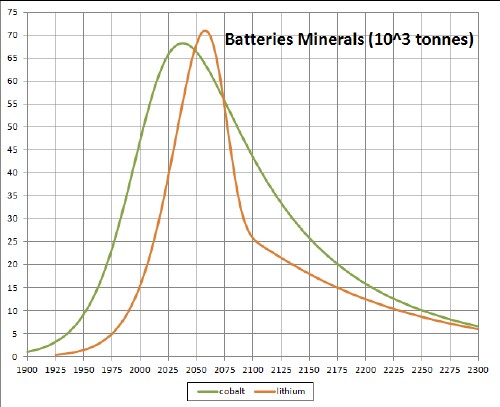
The following three graphs summarize the depletion/recycling curves for the ten batteries minerals:
 |
 |
 |
L. David Roper interdisplinary studies